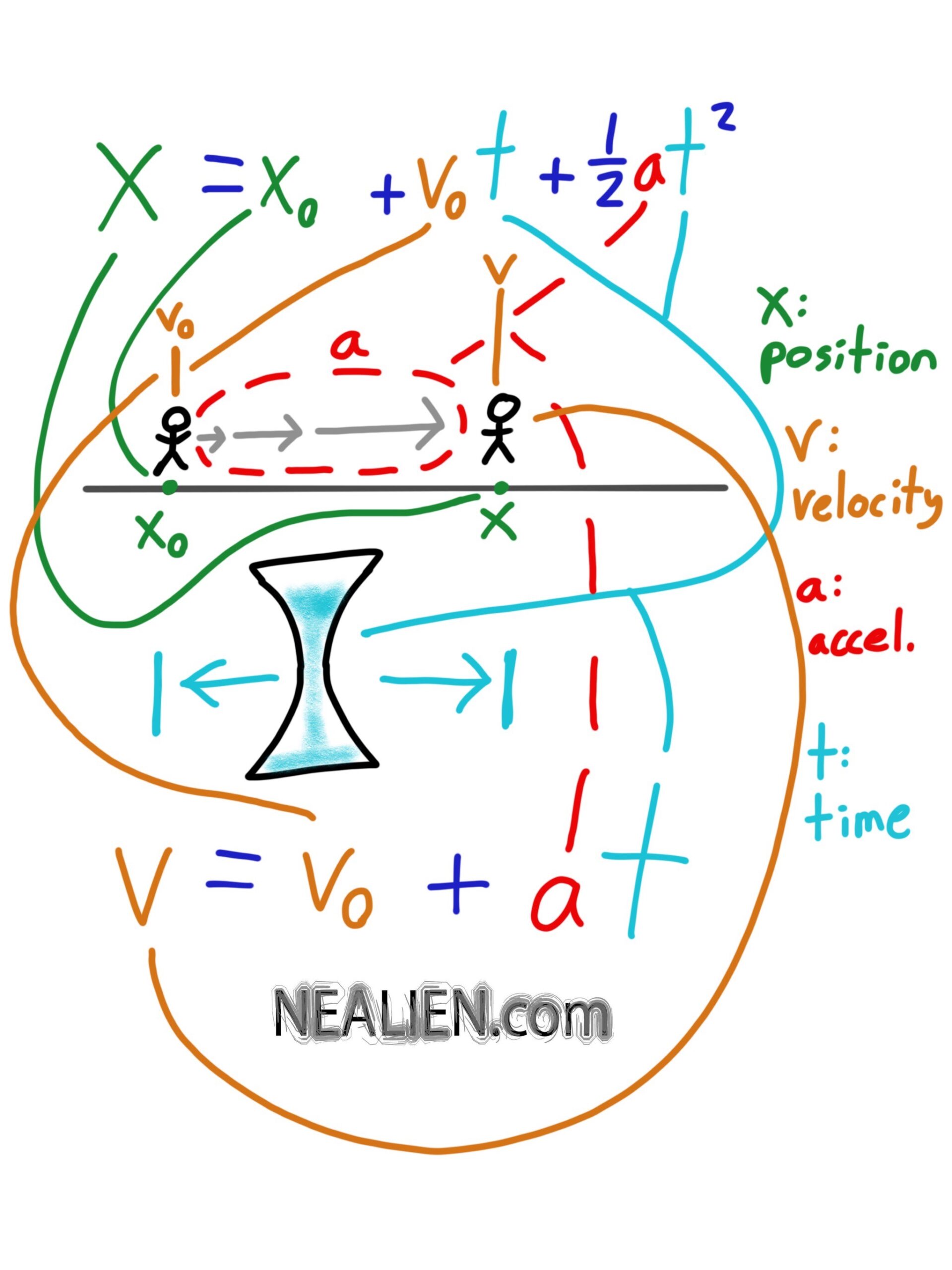
We looked at induced currents, loops, magnetic fields. Spent some time on the right hand rule. Also looked at the difference between dot products and cross products. They are important for the …
Continue Reading about Tutoring Physics, Dot Products and Cross Products Return →